Find the best Medical Software
Compare Products
Showing 1 - 20 of 1292 products
Sort by
Reviews: Sorts listings by the number of user reviews we have published, greatest to least.
Sponsored: Sorts listings by software vendors running active bidding campaigns, from the highest to lowest bid. Vendors who have paid for placement have a ‘Visit Website’ button, whereas unpaid vendors have a ‘Learn More’ button.
Avg Rating: Sorts listings by overall star rating based on user reviews, highest to lowest.
A to Z: Sorts listings by product name from A to Z.
athenaOne
athenaOne
The athenaOne Suite which includes athenaCollector a revenue cycle management solution and athenaClinials an EHR (electronic health records) recently ranked #1 in 2023 Best in KLAS for athenaClinials Ambulatory EMR for 11-75 physi...Read more about athenaOne
DrChrono
DrChrono
DrChrono’s iPad and iPhone compatible EHR and medical billing platform allows medical practices and healthcare providers to manage patient intake, patient care, clinical charting, billing and revenue cycle management. It includes ...Read more about DrChrono
RXNT
RXNT
RXNT’s cloud-based, ONC-certified medical software—Billing, Practice Management, EHR, and more—improves clinical outcomes & revenue cycle management. Simple, transparent pricing includes free setup and training, free data transfer...Read more about RXNT
AdvancedMD EHR
AdvancedMD EHR
AdvancedMD is a unified suite of software solutions designed for mental health, physical therapy and medical healthcare organizations and independent physician practices. Features include practice management, electronic health rec...Read more about AdvancedMD EHR
Talk with us for a free
15-minute consultationSoftware Advice is free because vendors pay us when they receive sales opportunities.
This allows us to provide comprehensive software lists and an advisor service at no cost to you.
This allows us to provide comprehensive software lists and an advisor service at no cost to you.
Meet Eric, a software expert who has helped 1,534 companies select the right product for their needs.
Talk with us for a free
15-minute consultationSoftware Advice is free because vendors pay us when they receive sales opportunities.
This allows us to provide comprehensive software lists and an advisor service at no cost to you.
This allows us to provide comprehensive software lists and an advisor service at no cost to you.
Tell us more about your business and an advisor will reach out with a list of software recommendations customized for your specific needs.
STEP 1 OF 4
How many doctors are in your organization?
ChartLogic EHR
ChartLogic EHR
ChartLogic offers an ambulatory EHR suite that includes electronic medical record, practice management, revenue cycle management, e-prescribing and patient portal. The solution caters to primary care, surgical care and other compl...Read more about ChartLogic EHR
PracticeQ
PracticeQ
PracticeQ is a secure and cloud-based practice management software that assists healthcare practitioners with onboarding, managing, and securing patient interactions. Teams can streamline booking, billing, and charting processes. ...Read more about PracticeQ
Elation Health
Elation Health
Elation Health is the most trusted technology platform for high-value primary care. Since 2010, the company has delivered clinical-first solutions — built on a collaborative EHR platform — that help practices start, grow, communic...Read more about Elation Health
Practice EHR
Practice EHR
Practice EHR, a medical practice management software, was developed to accommodate the needs of small to mid-size businesses. The platform is customizable to internal medicine practices, chiropractors, physical therapists, family ...Read more about Practice EHR
PrognoCIS
PrognoCIS
PrognoCIS EHR and PrognoCIS Telemedicine have earned a reputation for fast, flexible individual provider workflow. The software package offers a full suite of highly desirable features and functions. PrognoCIS provides a cloud-bas...Read more about PrognoCIS
MDnet EHR
MDnet EHR
Enable Healthcare presents a state-of-the-art electronic health records solution designed to transform the way doctors and providers manage patient data. Our AI-powered charting, powered by EnableAssist, revolutionizes charting ef...Read more about MDnet EHR
Nextech EHR & PM
Nextech EHR & PM
For more than 20 years, Nextech has provided a full-featured EMR and Practice Management solution within a single database. This system is a fit for dermatologists, plastic surgeons, ophthalmologists, and physicians, and is used b...Read more about Nextech EHR & PM
Compulink Healthcare Solutions
Compulink Healthcare Solutions
Compulink Advantage is an all-in-one database EHR solution for specialty practices such as optometry, ophthalmology, orthopaedics, ENT, mental health, podiatry, and more. Available cloud-based or server, Advantage includes smart f...Read more about Compulink Healthcare Solutions
WebPT
WebPT
Established in 2008, WebPT is the nation’s most trusted outpatient rehab therapy software platform in the country, helping more than 150,000 rehab therapy professionals from all practice sizes and specialties run successful and ef...Read more about WebPT
AestheticsPro
AestheticsPro
AestheticsPro is a cloud-based, HIPAA compliant medical spa management software solution that offers staff and calendar management along with client management, a point-of-sale and marketing tools within a suite. The staff manage...Read more about AestheticsPro
ModMed
ModMed
ModMed, also known as Modernizing Medicine®, is an award-winning software company that places doctors and patients at the center of care through an intelligent, specialty-specific cloud platform. Services include electronic health...Read more about ModMed
Myriad
Myriad
Myriad Health is a truly free practice management, billing and e-prescription platform for medical practices. Recognized as a no fraud healthcare business under Visa and MasterCard's healthcare program, these services are availabl...Read more about Myriad
Nexus EHR
Nexus EHR
Nexus EHR is an ONC Certified 2015 Edition Cures Update cloud-based ambulatory EHR and PM platform. It is designed for small to midsize practices and various specialties including orthopedics, neurology, podiatry, cardiology, gene...Read more about Nexus EHR
NewCrop
NewCrop
NewCrop is a comprehensive prescribing software solution that can be used with a wide variety of electronic medical systems. NewCrop is designed to integrate with any EMR or EHR system. NewCrop includes industry-leading functional...Read more about NewCrop
CharmHealth
CharmHealth
CharmHealth is a MU certified, cloud-based EHR, Practice Management and Medical Billing solution that helps healthcare organizations ranging from large multi-specialty groups to small independent medical offices function efficient...Read more about CharmHealth
Valant EHR Suite
Valant EHR Suite
Valant’s Behavioral Health EHR and Practice Management Software includes all the tools you need to provide exceptional individual and group care while running a successful private practice. Unlike other non-specialized EMR softwa...Read more about Valant EHR Suite
Popular Comparisons
Buyers Guide
Last Updated: November 21, 2023What is Medical Software?
Medical software is a broad term that includes any systems that help manage the clinical and administrative functions of healthcare organizations.
Systems have been tailored to automate just about every healthcare process, including billing, patient scheduling, creating and managing patient records, picture/image archiving, prescribing medication and more.
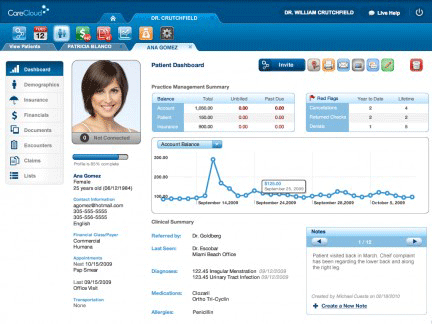
The Patient Dashboard in CareCloud, a modern, cloud-based system
In this guide we discuss the following:
Common Features of Medical Software
Popular Medical Software Comparisons
Common features of medical software
Electronic medical record (EMR) or electronic health record (EHR) software assists in creating and storing digital patient records. Helps track patient notes, demographics, histories and medications. Features include e-prescribing, SOAP notes, E&M coding advice and more. EMRs may also provide medical lab integration, device integration, tablet support and voice recognition. | |
Manages the creation of patient statements and submission of claims. Functions include coding, claim scrubbing, eligibility inquiry, electronic claim submission, payment posting and reporting. | |
Automates the process of scheduling patient visits. Features include automated follow-ups, text message/phone/email reminders and multi-location support. Typically offered with billing in a practice management suite. | |
Manages the operations and workflow of radiology imaging centers. Automates the process of storing, manipulating and distributing patient data and images. | |
Manages the storage and retrieval of DICOM images (X-rays, CAT scans, MRIs etc.). Often used in conjunction with an RIS to execute the radiology workflow efficiently. | |
Automates accounting procedures for healthcare practices. Functions include A/R, A/P, general ledger, financial reporting and more. | |
Combines practice management software and EMR software to handle the business and practitioner sides of a clinic. | |
Helps doctors and practices create, print, record and transmit prescriptions by offering a group of dedicated applications and software add-ons. | |
Allows doctors to stay in communication with their patients by providing educational resources and improving patient-provider relationships. | |
Tracks data for doctors and practices such as patient intake, revenue cycle, reimbursement rates, and other information to help give an understanding of overall operations. |
What type of buyer are you?
Most organizations we speak with are researching and evaluating medical software for one or more of the following reasons:
Transitioning from paper charts to digital records. “It’s raining paper” is the common cry we hear from paper-based practices. These buyers want to cut back on paper, improve office efficiency, reduce errors and run a more effective operation overall.
Replacing outdated software. This is a common scenario we hear from buyers. Their current system—whether it be a homegrown system or from a medical software vendor—is out of date and costly to maintain or update. They want a more modern system that is easier to use, meets federal requirements (e.g., ONC-ATCB certification) or that meets feature/functional needs.
Combining applications into an integrated suite. In many cases these practices have a hodgepodge of disparate applications, and as a result, find themselves doing double data entry and dealing with other inefficient workflows and processes. These organizations invest in integrated medical office management software—that is, integrated EMR, billing and scheduling applications—to centralize all information and functions in one place.
Implementing best-of-breed applications. Conversely, these buyers are focused on applications to address a specific need. Most often, buyers in this category are looking for a stand-alone billing, EMR, RIS or PACS system.
Pursuing federal incentives. Thanks to the HITECH Act of 2009, physicians have been replacing their EHRs or purchasing new ones for the first time to meet federal requirements. In order to qualify for Medicare and Medicaid incentives, physicians—or more accurately, “eligible professionals”—must make “meaningful use” of a certified EHR. The law offered incentives for physicians who complied before 2015, but physicians who still aren’t meeting “meaningful use” standards today face penalties in the form of decreased reimbursements.
We should note that outpatient and inpatient organizations often have different feature/functional requirements. For example, inpatient care provider centers such as hospitals will require systems to support bed management, UB-04 billing and potentially long-term patient stays. Meanwhile, ambulatory care providers such as primary care physicians and specialists will share common feature requirements to support “walk-in/walk-out” care.
Practices looking to integrate business intelligence tools into their existing medical solutions might be interested in healthcare BI software.
Benefits of medical software
The general benefits of any medical system are improved quality of patient care, increased operational efficiency and improved practice profitability. These benefits are created by different applications and impact organizations in different ways.
For example:
The automation of back-office operations streamlines administrative tasks associated with patient encounters, which may enable providers to spend more time with patients and hire fewer staff.
More accurate documentation of these encounters and a more organized claims submission process can lead to improved collections.
Automated alerts prompt providers with potential issues or risks, while automated reminders help patients return to the office when necessary, improving quality of care.
In addition to these general benefits, the major applications found in medical software each provide a host of specific benefits. For example, in 2014 we surveyed physicians about the benefits of electronic health records. They cited “easy access to records,” “more robust/legible records” and “drug interaction alerts” as the top advantages of using an EHR.
Important considerations
Integrated suite vs. best-of-breed. When selecting a system, buyers will have the choice of implementing different applications for specific tasks, or a complete suite of tools to address all their needs. The key decision that most providers will need to make is whether to implement a standalone electronic medical records (EMR) system or replace an existing practice management system with a complete system. We hear from many buyers facing this decision as practice management systems have been ubiquitous since the 1990s and EMRs are increasing in adoption, primarily due to the HITECH Act.
Software-as-a-Service (SaaS). The trend toward cloud computing is impacting many industries, and healthcare is certainly one of them. Web-based, or SaaS, software offers several advantages such as lower upfront costs, reduced IT and support costs, remote accessibility and more. However, practices in rural settings may not have access to the broadband Internet necessary to efficiently run Web-based software. Moreover, Web-based systems may not support all the feature/functional needs of some practices with unique requirements.
Mobile EHR Software. Going hand-in-hand with SaaS, healthcare providers are finding themselves increasingly on the go and accessing systems from multiple offices, home and mobile devices. Tablet (e.g., iPad) and smartphone support, including iPhones and Android phones, is increasingly common. If you will be accessing your software primarily from a mobile device, we suggest choosing a vendor that has developed a native app for your device, such as MediTouch’s iPad EMR.
ONC-ATCB certification. As most healthcare professionals are aware, the HITECH Act of 2009 requires the use of electronic medical records systems by 2015. Eligible professionals can subsequently qualify for up to $44,000 through the Medicare EHR Incentive Program or up to $63,750 through the Medicaid EHR Incentive Program. To qualify, they will need to demonstrate “meaningful use” of one of the ONC-ATCB certified EMRs.







